Copyright 2024 All rights reserved.
What We Do
By overcoming barriers that seemed impossible, we have established platform
technologies for development of pharmaceuticals that anyone can safely use regardless of time and place.
Overcoming Immune Rejection
What is Immune Rejection?
All cells contain antigenic molecules that can distinguish between self and non-self. These molecules are called Major Histocompatibility Complex (MHC), and in humans,
they are also called Human Leukocyte Antigen (HLA). Immune cells protect our body by recognizing MHC of non-self cells or foreign pathogens and removing them,
which is called immune rejection. Therefore, most currently approved cell therapy products are self-derived cells, and they are warned that immune rejection will occur if used on others.
“This medicine, autologous cells, should be used only on the patient himself,
because immune rejection and/or adverse immune reaction may occur if used on others.”
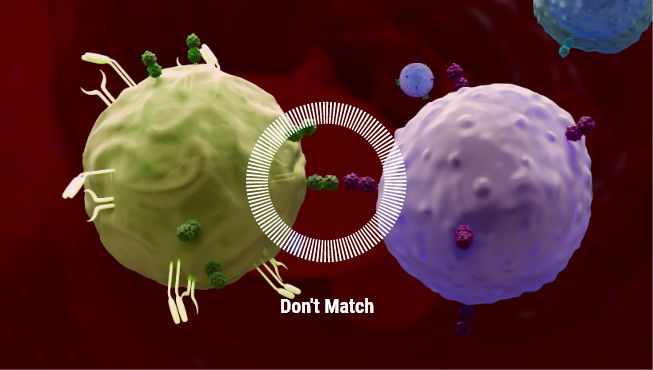
Immune Rejection
Immune Rejection :
Barrier to allogeneic cell therapy
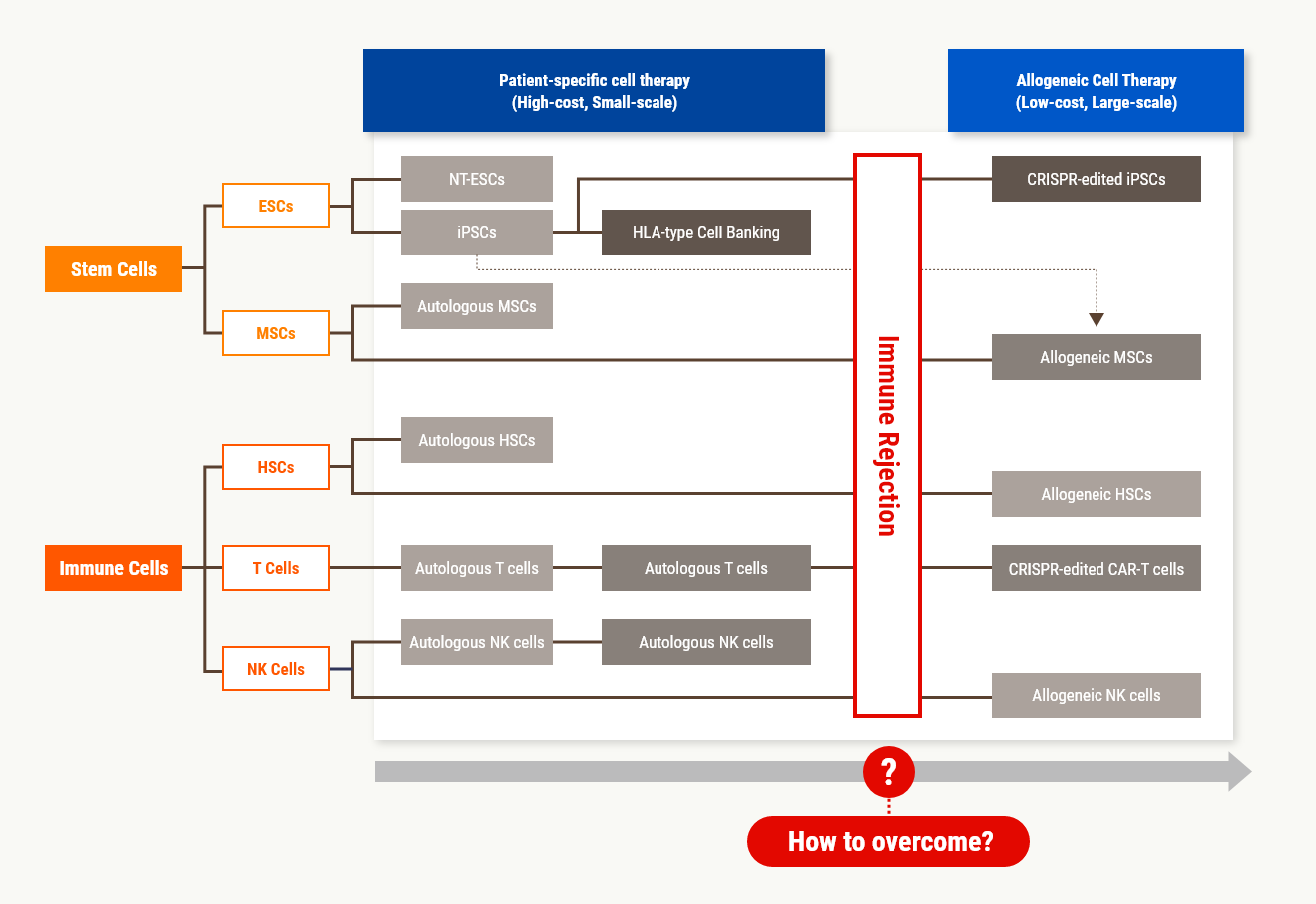
The recent issue of cell therapy products is shifting from the current patient-specific cell therapy products to the development of general-purpose cell therapy products that can be
allogeneically transplanted. However, these efforts are blocked by the barrier of immune rejection, which has not been overcome over the past 80 years.
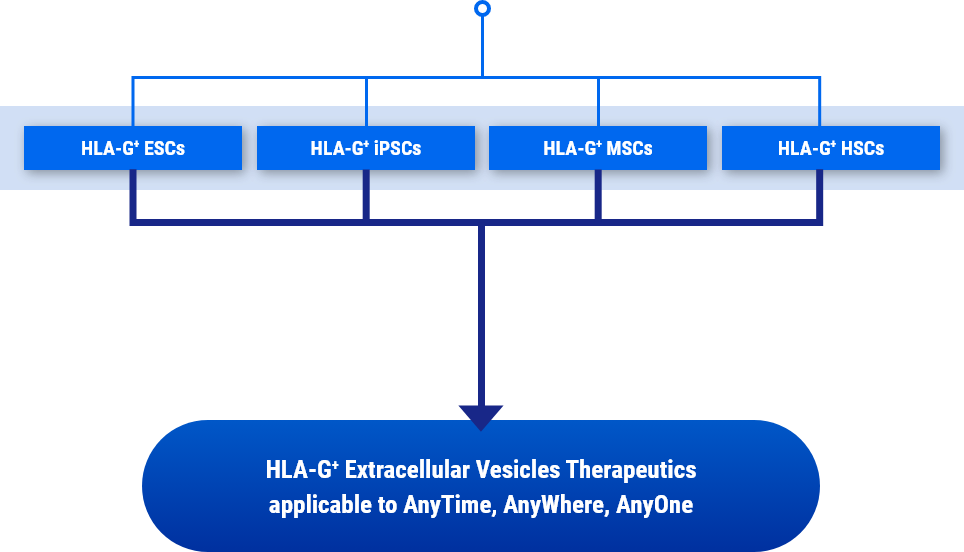
Stem Medicare overcomes this barrier and provides innovative technology to develop allogeneic cell therapy and furthermore, extracellular vesicle therapeutics, that anyone can safely use.
Key to overcoming Immune Rejection:
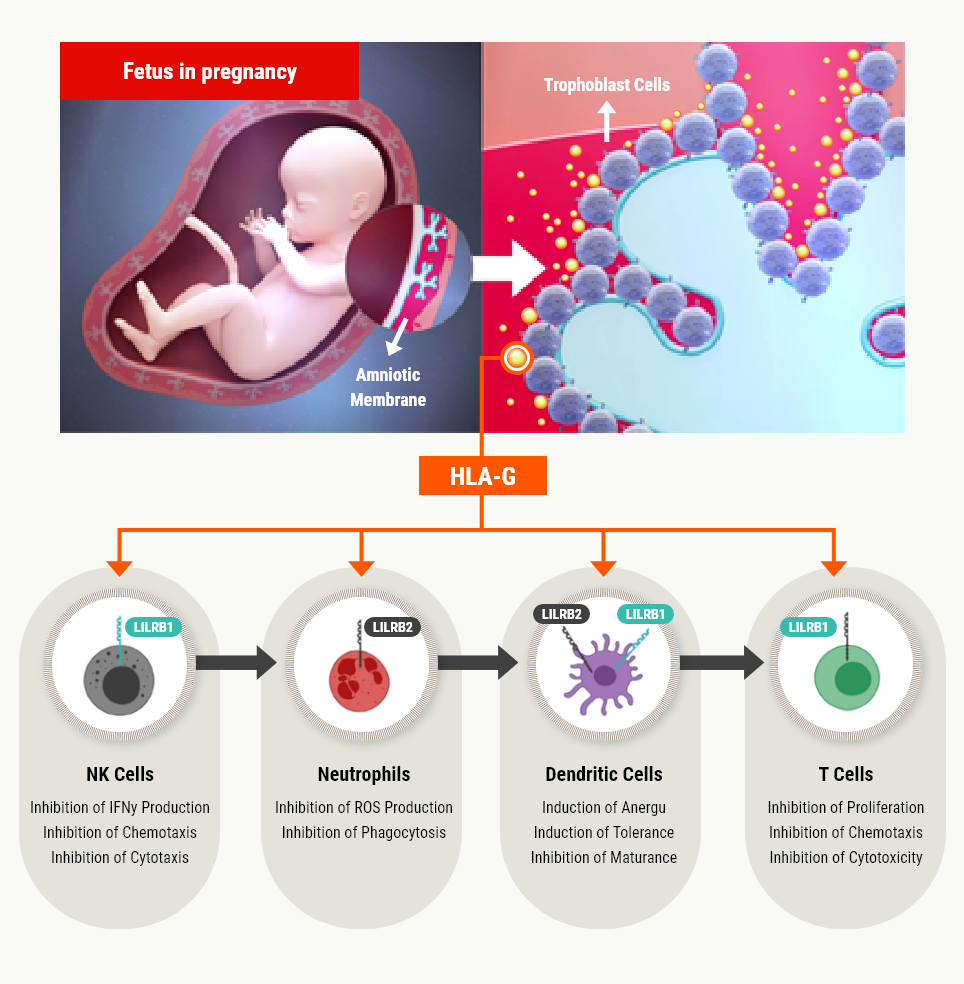
HLA-G (Human Leukocyte Antigen G) in pregnancy
Immune-tolerized
Extracellular
Vesicles
(HLA-G+ EVs)
(Journal of Extracellular Vesicles, 2020, vol. 9)

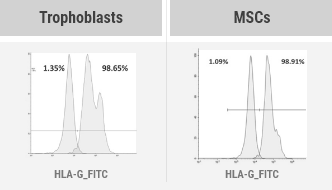
HLA-G, a nonclassical MHC-1 molecule, is secreted from trophoblasts under the stimulation of progesterone, a pregnancy hormone, and preferentially binds to the inhibitory receptors of maternal immune cells over other MHC-1 molecules, leading to induce an "immunotolerant environment" that protects the fetus (the embryo) from them. Therefore, it is known as a natural immunosuppressant without any side effect.
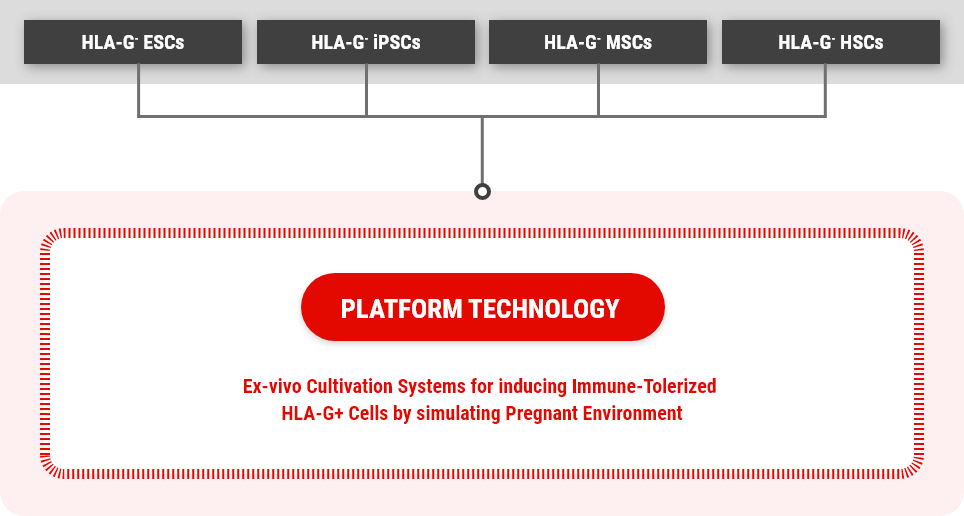
We developed an EX-VIVO culture system that simulates in vivo environment during pregnancy in which HLA-G secretion is induced only in trophoblasts, and through this system, we established the PLATFORM Technology that any cell line can be induced into the immune-tolerized
cell line expressing and secreting HLA-G.
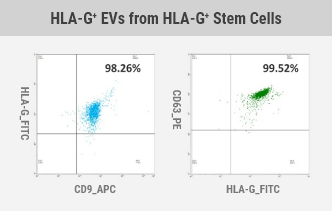
Ultimately, we have secured the world's first original technology to develop various allogeneic EV therapeutics without immune rejection by developing mass production technology for HLA-G-containing extracellular vesicles secreted from immune-tolerized cell lines, including stem cells (MSCs, HSCs, etc.) and immune cells).
Not applicable for
allogeneic use
(only useful for autologous)
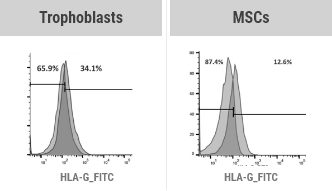 Weak HLA-G Expression
Weak HLA-G Expression
Applicable for allogeneic use
without immune rejection