Copyright 2024 All rights reserved.
Core Technology
We have developed and established all core technologies by ourselves
for the whole process from R&D to mass production of immune-tolerized EV therapeutics.
Mass Production of immune-tolerized EVs
Stemmedicare's cell culture platform technology is the only technology that can establish an immune-tolerized cell line with HLA-G expression and secretion characteristics regardless of cell origin. In addition, the immune-tolerized cell line expressing HLA-G is a cell line with significantly improved EV secretion ability as well as cell proliferation. Moreover, the EV isolation technology developed by Stemmedicare is also an innovative technology that can separate EVs with much higher purity and without any physical damage or impurities compared to commercial isolation methods.
Platform Technology for mass production of
HLA-G+ EVs not causing immune responses
-
OPTIONA
Ex-vivo culture system simulating
pregnancy environment
-
OPTIONB
Optimal Temperature Profile with
circulation for cell culture
-
OPTIONC
Serum-free 3D culture matrix for
improved cell proliferation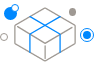
-
OPTIOND
EV isolation with higher purity than
commercial isolation methods