Copyright 2024 All rights reserved.
Pipelines
Stem Medicare is developing EV-based new drugs for various diseases
as well as regenerative medicines based on the world's only non-immunogenic,
immune-tolerized extracellular vesicle technology.
Atopic Dermatitis
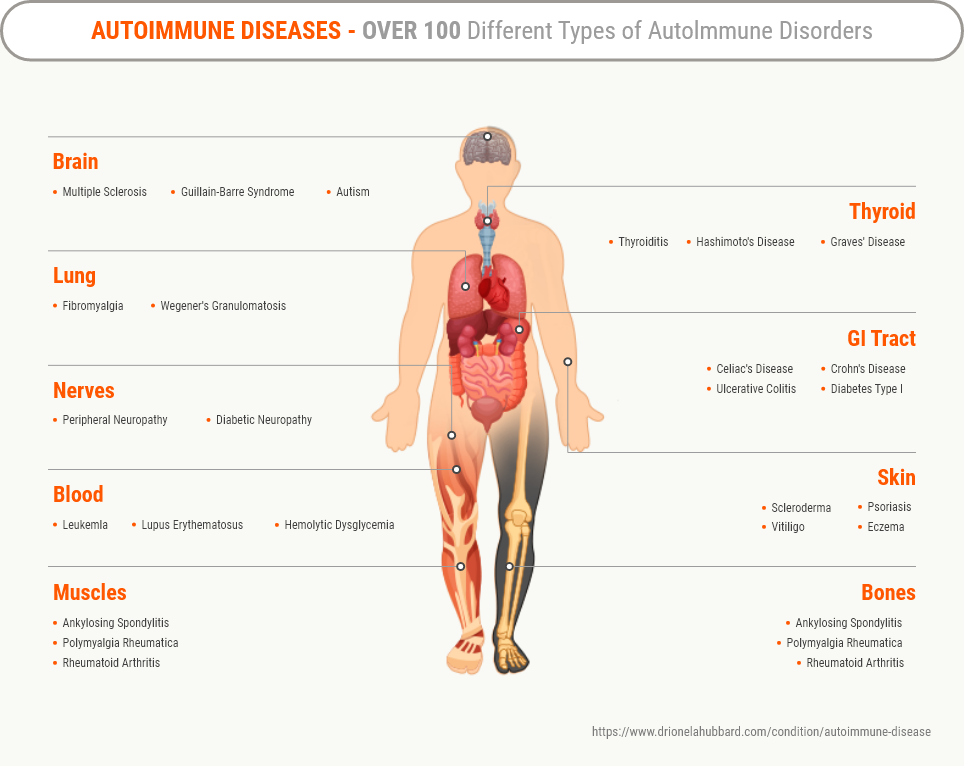
Atopic dermatitis is a type of autoimmune disease in which our immune cells cause hyperimmune reactions and destroy our own healthy tissues, including the heart, brain, nerves, muscles, skin, eyes, joint, lungs, kidneys, and blood vessels. Since the clinical phenotypes of atopic dermatitis vary greatly by race, age, and region, there is still no treatment that can be applied to all clinical phenotypes of atopic dermatitis.
The extracellular vesicle therapeutics (MBTC-AD-EVs) developed by Stemmedicare for the treatment of atopic dermatitis have the advantage of maintaining the homeostasis of the Th1/Th2 immune responses and being applicable regardless of the Th1/Th2/Th17/Th22 phenotype. Therefore, the indications of MBTC-AD-EVs are expected to be expanded as new therapeutics that can cure all autoimmune diseases (asthma, rheumatoid arthritis, Crohn's disease, type I diabetes, etc.) including atopic dermatitis.
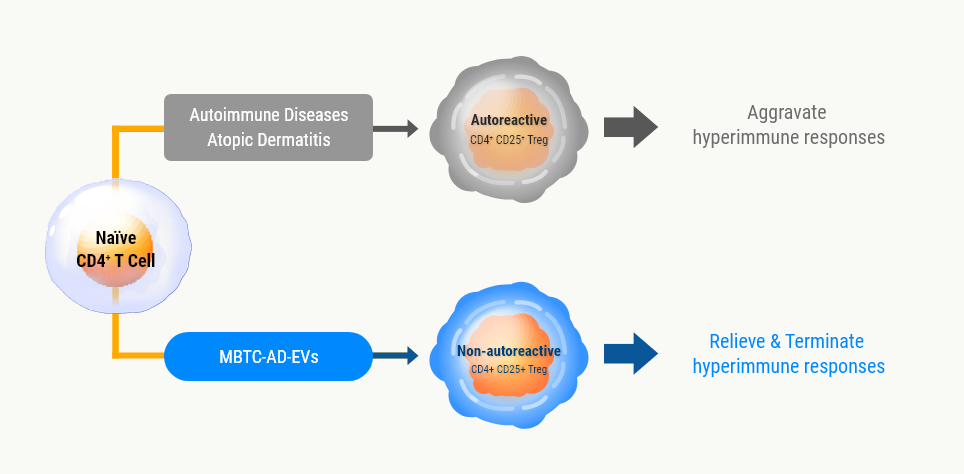
Mode of Action (MOA)
Promotion of
differentiation to
non-autoreactive
regulatory T cells
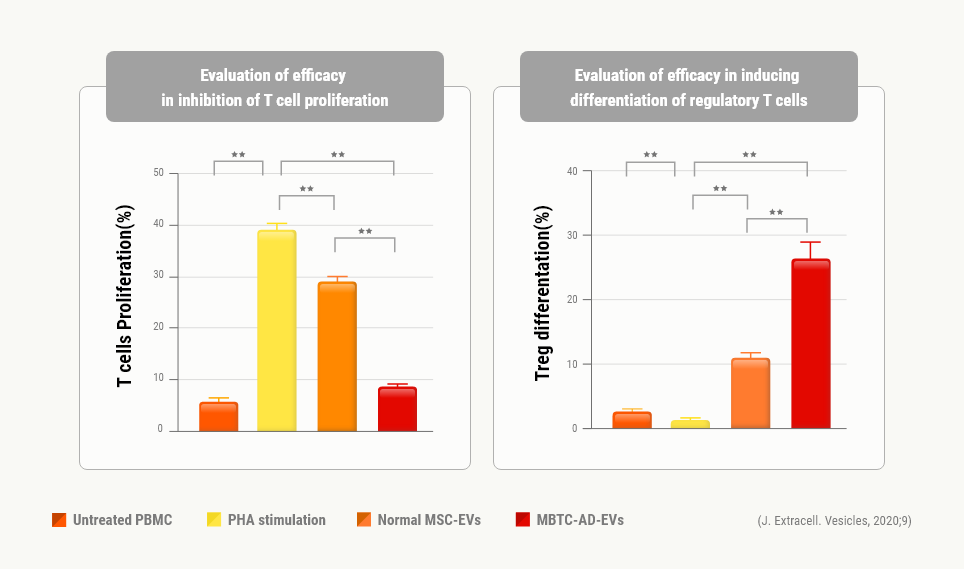
In vitro Assay
Inhibition of T cell
proliferation &
Induction of regulatory
T cell differentiation
In vivo Assay
Clinical Trials of
RETROPHA-G Cream
containing
MBTC-AD-EVs
Evaluation of improvement effects of skin barrier strengthening and itching relief due to dry skin performed by Korea Testing & Research Institute (n=22)
-
Skin Itch Improvement Rate
 VAS (Visual Analogue Scale)
VAS (Visual Analogue Scale)Skin itch was significantly decreased after
4 weeks RETROPHA-G Cream
use as compared with before use. -
Skin Moisturizing Improvement Rate
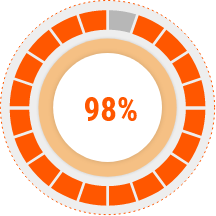 Corneometer
CorneometerThe skin moisturizing was significantly
increased after 4 weeks RETROPHA-G
Cream use as compared with before use
Evaluation of the efficacy and safety of RETROPHA-G Cream on atopic dermatitis performed
by Cheonan Oriental Hospital of Daejeon University (n=30, 4 weeks use)
-
Skin Itch
Improvement Rate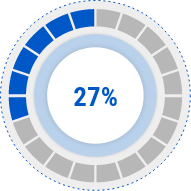
-
EASI Score
Decrease Rate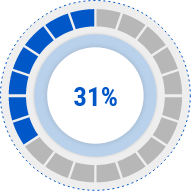
-
Skin Moisturizing
Improvement Rate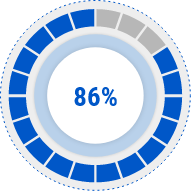
-
Serum IgE
Decrease Rate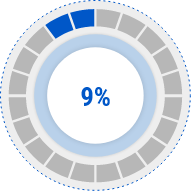
MBTC-AD-EVs
-

Immune-tolerized extracellular vesicle
therapeutics for atopic dermatitis -

Instant skin itching relief and
skin barrier strengthening effects -

Applicable to all clinical phenotypes of atopic
dermatitis due to innovative MOA of
promoting differentiation to non-autoreactive
regulatory T cells -

Applicable to atopic dermatitis of all ages and
grades without any side-effect -

Applicable to the treatment of all autoimmune
diseases including atopic dermatitis -

New Excellent Technology (NET) Certificated