Copyright 2024 All rights reserved.
Core Technology
We have developed and established all core technologies by ourselves
for the whole process from R&D to mass production of immune-tolerized EV therapeutics.
Core Technology
FBS & DMSO-free Cryopreservation
Cell Cryopreservation Process
The process of stable preservation of cell lines at cryogenic temperatures to minimize genetic mutations, prevent aging and transformation, and avoid loss due to contamination
(essential process for development and production of advanced biopharmaceuticals)
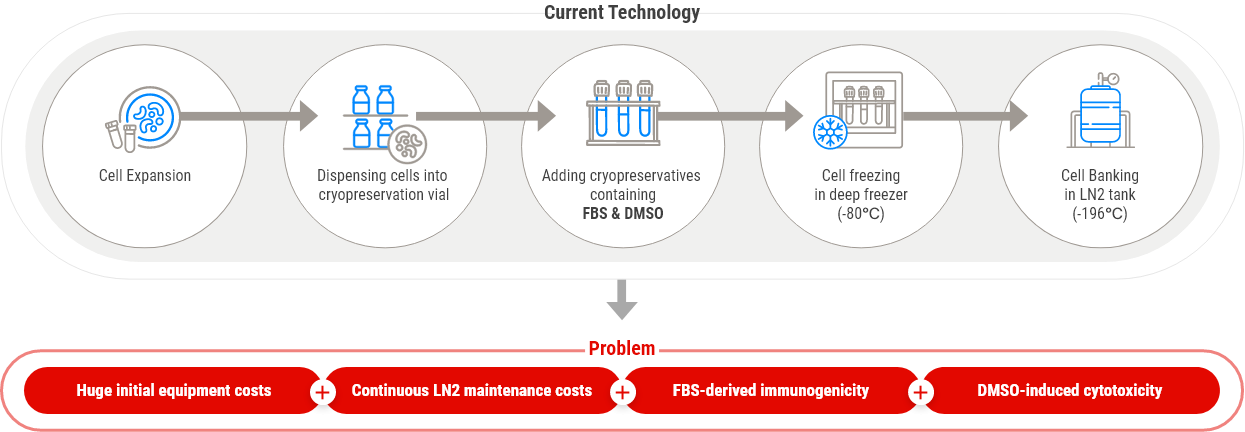
Biohazards of cryopreservatives containing FBS and DMSO
-
FBS(Fetal Bovine Serum)
- Risk of contamination with bovine pathogens, such as bovine viruses, prions, mycoplasma, or other infectious agents causing BSE (mad cow disease)
- Internalized animal antigens in cryopreserved cells may cause harmful immune reactions in patients for clinical application
- Entirely dependent on imports and no domestic alternative technology
Immunogenicity / Tumorigenicity
Infection with bovine-derived diseases -
DMSO(Dimethyl Sulfoxide)
- Induces DNA damage, transformation, and apoptosis of
cryopreserved cells by cytotoxicity - DMSO Cryopreserved Stem cell transplantation causes side effects
(allergies, gastrointestinal disorders, neurological disorders, etc.) - Adverse reactions occurred in 67% of patients injected with
DMSO cryopreserved hematopoietic stem cells (HSCs) - Entirely dependent on imports and no domestic alternative technology
Cytotoxicity / Gene Alteration
Not recommend for embryo and egg storage - Induces DNA damage, transformation, and apoptosis of
MBTC-CryoGuardTM (FBS-free, DMSO-free, -80℃ applicable)
World’s first cryopreservation agent containing immune-tolerized stem cell-derived extracellular vesicles replacing FBS and DMSO
-
Safety
- Do not contain any animal-derived component, serum component, and DMSO
- Including immune-tolerized stem cell-derived EVs (HLA-G positive) not causing immunogenicity
- Securing the safety of final products (biopharmaceuticals) and eliminating concerns about side effects
-
Bio-activity
- Improved cell viability, proliferation rate, and biological activity after thawing of cryopreserved cell lines at -80℃ compared to those cryopreserved with FBS and DMSO in LN2 tank
- Contribute to increasing productivity and improving efficacy of final products (biopharmaceuticals)
-
Economics
- Do not need huge equipment cost for LN2 tank and maintenance cost
- Up to 98% reduction in cryopreservation costs for cell lines
- Contribute to securing price competitiveness of final products (biopharmaceuticals)
-
Availability
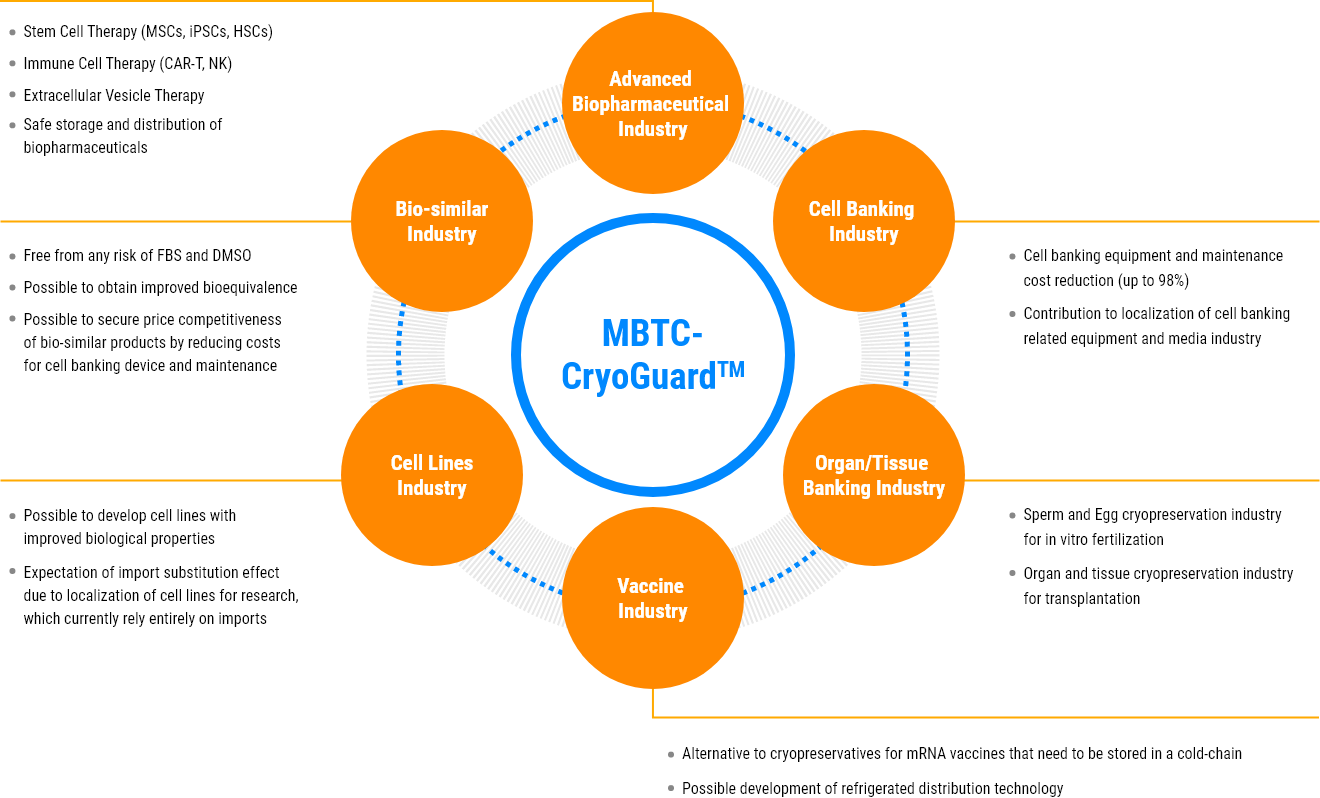
- Cryopreservation agent for cell therapy products : MSCs, iPSCs, HSCs, immune cells (CAR-T/NK/DC), etc.
- Cryopreservation agent for Cell Banking for biopharmaceutical manufacturing and R&D: RCB, MCB, WCB
- Cryopreservation agent for cell & tissue banking : Egg & Sperm Bank, HSC & Bone marrow bank, tissue and organ bank for transplantation, etc.
- Cold Chain for biopharmaceuticals including vaccines and tissue/organ for transplantation
Application